Transcriptomic similarities and conserved genes between human periodontitis and mouse ligature-induced periodontitis: A secondary analysis of gene expression omnibus datasets
Article information
Abstract
Purpose
This study aimed to evaluate how well the ligature-induced periodontitis mouse model reflects the transcriptomic features of human periodontitis and to identify periodontitis-associated genes and functions conserved across species.
Materials and Methods
RNA sequencing data from human and mouse gingival tissues were obtained from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were identified using DESeq2. Functional enrichment analysis was performed using Gene Ontology and KEGG pathway databases. Cross-species transcriptomic similarity was evaluated by comparing DEG overlap and enrichment similarity between human and mouse.
Results
A total of 223 human DEGs and 622 mouse DEGs were identified. Among these, 25 DEGs were shared between species, including 23 showing concordant regulation direction. The mouse-to-human overlap ratio was 3.70%. Functional enrichment analysis identified 189 significant terms or pathways in humans and 602 in mice, with 129 shared results. Specifically, 41.18% of mouse KEGG pathway results overlapped with human results. The shared concordant genes, including IL-1β, PTGS2 (also known as COX-2), and MMP13, were associated with immune and inflammatory functions.
Conclusion
The ligature-induced periodontitis mouse model reflects the transcriptomic features of human periodontitis in a limited manner, showing low similarity at the DEG level and moderate similarity at the enrichment level. Conserved DEGs such as IL-1β, PTGS2, and MMP13 may represent fundamental molecular mechanisms of periodontitis.
Introduction
Periodontitis is a chronic inflammatory disease characterized by the destruction of periodontal tissues, potentially leading to tooth loss [1]. Various animal models were utilized to investigate the pathophysiological mechanisms of human periodontitis [2]. Among these, the ligature-induced periodontitis mouse model was widely used due to its genetic similarity to humans, cost-effectiveness, and ease of handling [3]. Histological evaluation of ligature-induced periodontitis in mice showed prominent inflammatory responses from approximately 4 days after ligation, progressively increasing until day 8, after which no significant changes were observed [4]. In mice, periodontitis was also induced by injecting lipopolysaccharide or Porphyromonas gingivalis [5]. However, these methods required 4–6 weeks to produce significant periodontal tissue destruction, which was much longer than the ligation method [6]. Therefore, the ligature-induced periodontitis mouse model was effectively used for studying periodontal tissue destruction-related changes.
In addition, numerous studies used the ligature-induced mouse model to explore the molecular characteristics of periodontitis [6-8]. Differentially expressed genes (DEGs) in ligature-induced periodontitis were significantly enriched in functions such as neutrophil chemotaxis and inflammatory responses, and the expression of innate immune response-related genes S100a8 and S100a9 was markedly increased at both RNA and tissue levels in periodontitis [7]. Substance P played a key role in regulating inflammation and alveolar bone loss in ligature-induced periodontitis, whereas calcitonin gene-related peptide had a minimal effect [8]. Through such studies, the molecular characteristics of periodontitis were elucidated using the ligature-induced periodontitis mouse model. However, to the best of our knowledge, no study has directly evaluated how similar the molecular profiles observed in the ligature-induced periodontitis mouse model are to those in human periodontitis. In other words, it remains unclear to what extent this mouse model reflects the gene expression features of human periodontitis.
The aim of this study was to evaluate how well ligature-induced periodontitis in mice reflects the transcriptomic features of human periodontitis and furthermore to identify conserved periodontitis-related genes and their functions across species.
Materials and Methods
The overall workflow of this study is presented in Figure 1.
Data collection and preprocessing
RNA sequencing data from both human and mouse gingival tissues were obtained from the publicly accessible Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). Human data were retrieved from GSE223924, including 10 healthy gingiva samples and 10 periodontitis gingiva samples. All human subjects were systemically healthy. Periodontitis cases corresponded to stage II and III disease, classified according to the 2017 World Workshop criteria, while patients with Grade C (rapid progression or early onset) were excluded. Inflamed gingival tissues were obtained during open-flap surgery at diseased sites, and healthy tissues were collected during crown-lengthening or orthodontic extractions. Mouse data were retrieved from GSE184556, including 3 healthy gingiva samples and 3 ligature-induced periodontitis samples. In this dataset, periodontitis was induced by placing a ligature around the second maxillary molar, and gingival tissues were collected at 8 days post-induction. Three age- and sex-matched mice were used per group. In both groups, gingival tissues were harvested from the maxillary right molar region. Detailed characteristics of each sample can be found in the series matrix files available in the corresponding GEO entries. Raw count data were downloaded, and ortholog mapping between species was performed using Ensembl BioMart (Ensembl, European Molecular Biology Laboratory–European Bioinformatics Institute [EMBL–EBI], Hinxton, Cambridge, UK) in R (version 4.4.3; R Foundation for Statistical Computing, Vienna, Austria).
Differential gene expression analysis
Differential expression analysis was performed independently for the human and mouse datasets using the DESeq2 package (version 1.46.0) in R. Genes with an absolute log2 fold change ≥ 1 and a Benjamini-Hochberg adjusted p-value < 0.05 were defined as DEGs.
Functional enrichment analysis
Functional enrichment analysis was conducted using g:Profiler (version e113_eg59_p19_f6a03c19, https://biit.cs.ut.ee/gprofiler/). Analyses included Gene Ontology (GO) categories — Molecular Function (MF), Biological Process (BP), and Cellular Component (CC) — and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Significance was determined using the g:SCS-adjusted p-value provided by g:Profiler, with values < 0.05 considered significant.
Cross-species similarity analysis
To evaluate similarity between human and mouse DEGs, the mouse-to-human overlap ratio and the Jaccard similarity index were calculated. The mouse-to-human overlap ratio was defined as the proportion of mouse DEGs that were also identified as human DEGs. The Jaccard similarity index was calculated as the size of the intersection divided by the size of the union between the human and mouse DEG sets. In addition, similarity between the functional enrichment results derived from human and mouse DEGs was assessed using the same indices.
Results
Cross-species similarity of periodontitis-associated DEGs between human and mouse
Periodontitis-associated DEGs totaled 223 in humans and 622 in mice. Of these, 25 DEGs were shared between the two species, including 23 shared concordant DEGs showing the same regulation direction. Shared concordant DEGs accounted for 3.70% of mouse DEGs, and the Jaccard similarity was 2.80%.
Cross-species functional similarity based on periodontitis-associated DEGs
Functional enrichment analysis based on DEGs identified 189 significant GO terms or KEGG pathways in humans and 602 in mice (Table 1). Among these, 129 terms or pathways were shared between the two species. Overall, 21.43% of the mouse results were shared with human results, and the Jaccard similarity was 19.49%. Specifically, 41.18% of the KEGG pathway results from mice were shared with humans, while 9.52% of the GO:MF terms overlapped.
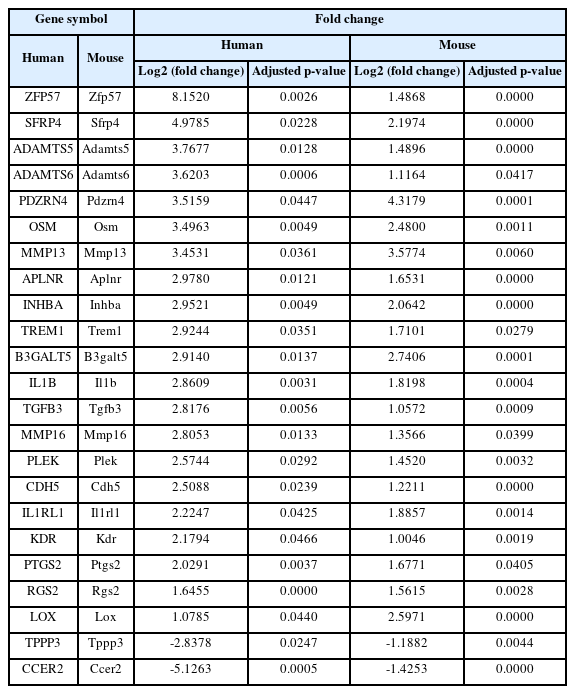
Functional and network analysis of shared concordant DEGs between human and mouse
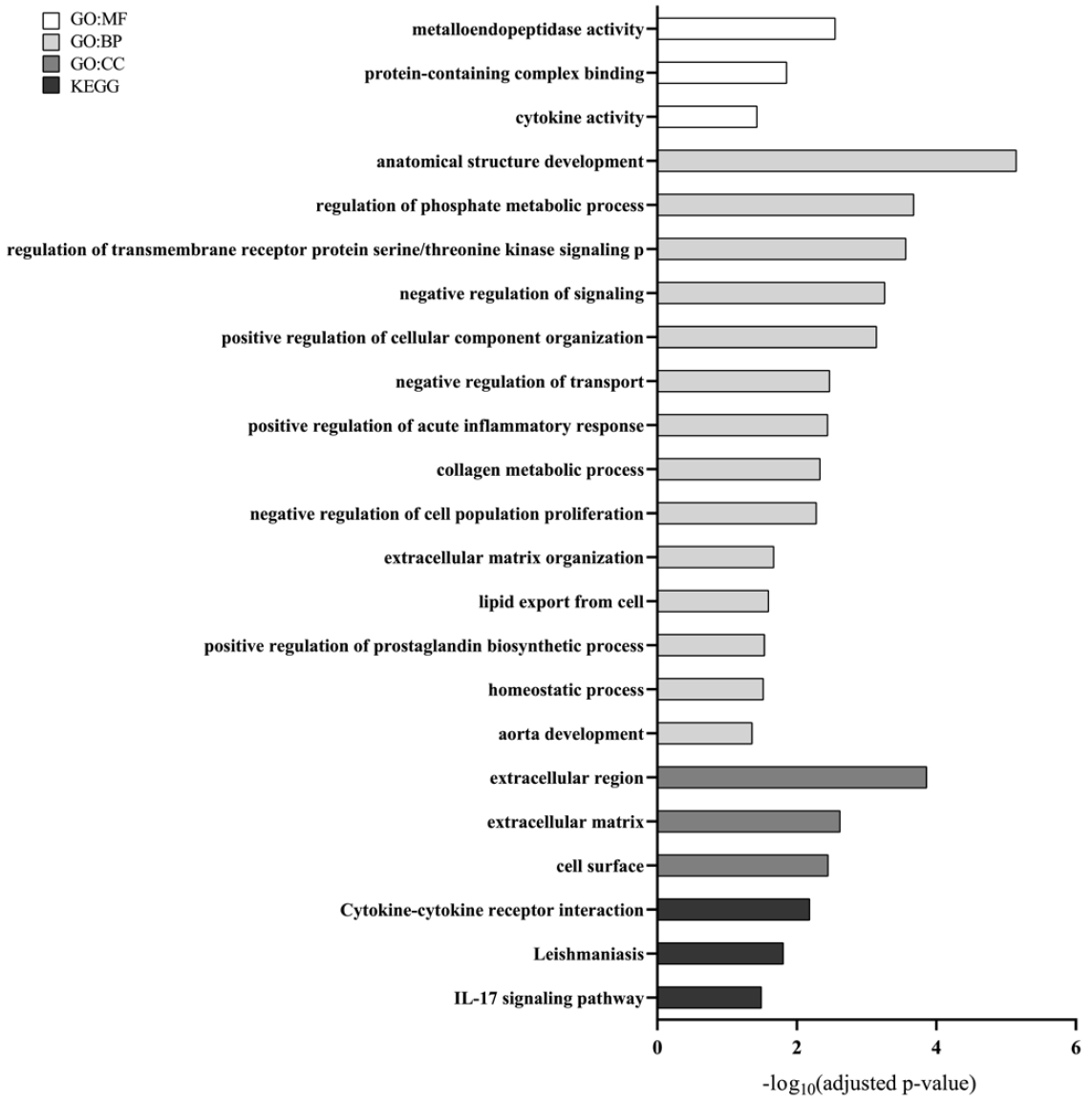
A total of 23 shared concordant DEGs between human and mouse were identified (Table 2). Protein–protein interaction (PPI) relationships among these genes in human were assessed and visualized (Fig. 2). IL-1β showed the highest node degree, followed by PTGS2, LOX, and MMP13. ZFP57 and CCER2 exhibited the largest absolute log₂ fold change, while both had a node degree of 0. Functional enrichment analysis of the shared concordant DEGs identified 79 GO terms (3 MF, 68 BP, 8 CC) and 3 KEGG pathways as significantly enriched (Fig. 3; only the driver terms are shown for GO).

Protein–protein interaction networks. The five genes with the highest node degrees are IL1B (11), LOX, MMP13, and PTGS2 (each 7), and KDR (6).
Discussion
To our knowledge, this is the first study to analyze DEGs in both human and mouse periodontitis. This study provides evidence for evaluating whether the ligature-induced periodontitis mouse model is suitable for exploring transcriptomic features of human periodontitis. We also identified periodontitis-associated DEGs conserved between human and mouse and characterized their functions.
In the present study, the mouse-to-human overlap was 3.70% at the DEG level and 21.43% at the functional enrichment level, indicating that the ligature-induced periodontitis mouse model only partially reflected human periodontitis transcriptomic features. Meanwhile, 41.18% of the KEGG pathway results from the mouse overlapped with those from humans. This suggested that the ligature-induced periodontitis mouse model might have more effectively reproduced pathway-level functions than individual DEGs. A previous study in peri-implantitis conducted a similar analysis, performing RNA sequencing using a ligature-induced peri-implantitis mouse model and comparing the results with those from humans [9]. It reported a mouse-to-human overlap of shared concordant DEGs of 28.63% and a Pearson correlation coefficient of gene expression patterns of 0.6935. Taken together with our findings, this suggested that despite inducing inflammation in the periodontal tissue using a similar method, the transcriptomic reproducibility of the mouse model was higher in peri-implantitis than in periodontitis. This might have been attributed to the greater differences in tooth structure and morphology between humans and mice [10], compared with those in implant fixtures produced through standardized manufacturing processes.
A total of 23 shared concordant DEGs were identified between human and mouse periodontitis, showing consistent regulation direction across species. These shared concordant DEGs might have represented conserved molecular responses and underlying mechanisms of periodontitis, potentially playing a more fundamental role in the disease than other DEGs. Among them, IL-1β and PTGS2 were well-established periodontitis-associated genes. IL-1β, which showed the highest node degree in this study, is known as a key gene in the onset and progression of periodontitis [11]. PTGS2, also known as COX-2, was reported to be highly expressed in inflamed periodontal tissues [12] and played a critical role in periodontal inflammatory responses [13]. Our findings supported the notion that these genes were central regulators of periodontitis and suggested that they were involved in fundamental periodontitis-related biological responses conserved across species. In addition to IL-1β and PTGS2, among the remaining 21 shared concordant DEGs, ZFP57 [14], ADAMTS5 [15], OSM [16], MMP13 [17], APLNR [18], and TREM1 [19] were reported as potentially associated with periodontitis. The other 13 genes had not been clearly linked to periodontitis in previous studies. PPI analysis of the 23 shared concordant DEGs revealed significantly greater interactions compared to random protein sets of the same size. Furthermore, these genes were significantly enriched in GO terms related to immune responses and inflammation, such as metalloendopeptidase activity, positive regulation of acute inflammatory response, and regulation of MAPK cascade. They were also significantly enriched in the IL-17 signaling pathway, which is known to play a key role in periodontitis [20]. These findings suggested that the shared concordant DEGs might have regulated periodontitis through these biological functions, and further studies were needed to clarify their specific roles individually or collectively.
This study conducted a comparative analysis of DEGs identified by comparing healthy and periodontitis samples in both ligature-induced periodontitis mouse models and human subjects. Our findings are significant as one of the few attempts to evaluate how well the ligature-induced periodontitis mouse model reflected the transcriptomic features of human periodontitis. Furthermore, this study identified periodontitis-related DEGs that were conserved between mice and humans.
However, there were limitations in that this study utilized publicly available datasets from GEO, and the sample sizes for both mouse and human data were relatively small. In addition, because the datasets were derived from independent studies, the number of samples differed between species. Future studies with larger and more balanced sample sets are warranted to validate the cross-species comparisons. Moreover, it is important to assess whether this varies with the method and duration of periodontitis induction, to develop more relevant mouse models. In particular, comparative studies with other induction methods, such as lipopolysaccharide injection or models induced by periodontal pathogens like Porphyromonas gingivalis, are needed to further clarify their translational relevance. Also, there is a need to verify whether the conserved periodontitis-related DEGs identified in this study are consistently observed across different datasets and to clarify their specific roles in the pathogenesis of periodontitis. This study would serve as a starting point for such efforts and contributes to a fundamental understanding of the molecular mechanisms involved in the onset and progression of periodontitis.
Notes
Conflicts of Interest
None
Acknowledgment
Sincere thanks to Professor Hyun-Joo Kim for her kind advice and encouragement.