Impact of COVID-19 Outbreak and its Different Waves on Hospital Admissions for Odontogenic Infections: A Single-Center Retrospective Study
Article information
Trans Abstract
Purpose
Odontogenic infections, which originate from teeth or their surrounding structures, are prevalent in the head and neck regions. The COVID-19 pandemic has altered healthcare-seeking behaviors due to policies adapted to impede the spread of virus, potentially impacting the management and severity of odontogenic infections. This study aims to investigate changes in characteristics of patients admitted to the hospital suffering from odontogenic infections before and after the COVID-19 outbreak.
Patients and Methods
A retrospective study was conducted on patients admitted to the Department of Oral and Maxillofacial Surgery at Dankook University Hospital from March 2017 to February 2023. Patients were divided into two groups based on pre and post-COVID-19 outbreak (Group 1 and Group 2, respectively). Clinical parameters, treatment modalities were compared between groups. Additionally within Group 2, COVID-19 positive and negative patients were compared, and Group 2 was subdivide into five groups according to different waves of COVID-19 outbreak.
Results
Following the COVID-19 outbreak, there was a significant increase in hospital admissions odontogenic infections. Group 2 exhibited higher severity scores, affected spaces, and surgical interventions compared to Group 1 patients. COVID-19 positive patients demonstrated elevated severity parameters. Subgroup analysis within Group 2 revealed the highest severity during the second wave of the COVID-19 outbreak.
Conclusion
COVID-19 outbreak correlated with a rise in hospital admissions and severity of odontogenic infections. Thus, healthcare providers should anticipate changes in odontogenic infection patterns during pandemics and adapt management strategies accordingly.
Ⅰ. Introduction
Odontogenic infections are those infections that originate in the teeth or their surrounding structures, and are the most common type of head and neck infections [1]. Odontogenic infections normally start out in a rather localized form but can spread to adjacent structures, usually via fascial spaces of the head and neck area. Once spread to fascial spaces, odontogenic infections may become severe, and special management such as surgical intervention, administration of antibiotics, and hospital admissions should be considered. Although the mortality rate related to odontogenic infections has dropped significantly owing to the advancement of antibiotics and improved surgical techniques, odontogenic infections still cause a variety of complications to the patient, such as airway obstruction, orbital abscess, cerebral abscess, descending necrotizing mediastinitis, necrotizing fasciitis, cavernous sinus thrombosis, sepsis, and Lemierre’s syndrome [2,3]. Several parameters have been used to determine the severity of odontogenic infections. C-reactive protein(CRP) levels, white blood cell(WBC) counts, lengh of hospital stay, use of different treatment modalities, number of affected fascial spaces, and severity score suggested by Flynn et al. are routinely used to assess the severity of infection [4,5]. Among the factors that affect the severity of odontogenic infections, accessibility to public dental care has an effect on how severe the symptoms are and the overall number of patients. According to a study conducted by Bowe et al., as public healthcare benefits gradually decreased, both the severity and number of odontogenic infections increased in the United Kingdom [6]. This result was consistent with a study performed by Salomon et al. at the University of Illinois in which, after restricting public dental care benefits, the severity and volume of odontogenic infections increased [7].
COVID-19 viruses are enveloped, non-segmented, and positive-sense RNA viruses that were first reported in Wuhan, China. Since then, it has rapidly become a global pandemic [8]. The virus was first reported on January 20th, 2020, in the Republic of Korea. In an effort to slow the diffusion of the virus, the Korean government issued social distancing measures starting on February 29th, 2020 [9,10]. Social distancing measures have caused foot traffic in public areas plummet by 13~25.5%, according to a policy report conducted by The Seoul Institute in 2021 [11]. Notably, the number of patients visiting private dental clinics has declined drastically, reaching 76% in the US. Similarly, a retrospective study conducted in 2020 by Lee et al. showed a significant decrease in the number of patients visiting dental clinics between February and April 2020 [12]. Since the frequency of patients visiting dental clinics declined due to fear of viral infection, proper treatment of rather simple dental-related infections were considered frivolous. A study performed in the United Kingdom by Samara et al. reported an increase in the severity and number of odontogenic infections, which corresponds to a study by Grill et al. in Germany [13,14,15]. In addition, a study reported that COVID-19 might lead to opportunistic infections with other viruses, bacteria, and fungi, which could potentially complicate preexisting conditions, including odontogenic infections [16].
Since only few studies on this topic have been conducted in Korea, the purpose of this study was to compare hospital admissions for odontogenic infection before and after the outbreak COVID-19 and according to different waves of virus spread since social distancing policies changed consequently.
Ⅱ. Materials and Methods
1. Study Participants
This retrospective study included all patients admitted to the Department of Oral and Maxillofacial Surgery at Dankook University Hospital between March 2017 and February 2023. Patients whose infection did not originate from odontogenic causes, those who did not show any clear clinical symptoms or had no to minimal swelling were excluded. Consequently, 12 patients were excluded and 348 patients were included. This study was approved by the institutional review board of Dankook University Dental Hospital (IRB number: DKUDH IRB 2024-03-002).
2. Methods
The groups were divided as of February 29th, 2020, which was the first day of implementation of social distancing policies. Group 1 comprised patients before social distancing and served as the control group. Group 2 comprised patients who underwent social distancing. A second study was conducted in Group 2, further dividing the group according to the different waves of the COVID-19 outbreak as described by the Korea Centers for Disease Control & Prevention(KCDC) [16].
Charts were reviewed for every patient, and age, sex, comorbidities, admission and discharge dates, initial CRP and WBC counts, treatment modalities, and COVID-19 test results were recorded. Other parameters that defined the severity of infection such as intensive care unit(ICU) admission blood culture results, number of surgeries performed, fasciitis, and mortality were also recorded. A single researcher reviewed the CT images taken on admission and the affected spaces, and severity score by Flynn et al. was recorded.
Finally, to determine whether COVID-19 infection affects the severity of odontogenic infection, a comparison between COVID-19 positive and negative patients was performed.
3. Statistical Analysis
The recorded data were entered in to Excel spreadsheets(Microsoft Office), and statistical analyses were conducted using IBM SPSS Statistics for Windows Version 29.0.1(SPSS Inc., Chicago, IL, USA). Statistical significance was determined using independent t-tests and one-way analysis of variance(ANOVA) for numerical values and Chi-square tests for comparison of distribution of non-numerical values. Statistical significance was set at P-values less than 0.05. Tables were created using Excel(Microsoft Office).
Ⅲ. Results
1. Study Participants
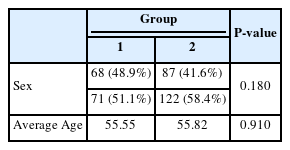
Group 1 comprised 68 females(48.9%) and 71 males(51.1%) with an average age of 55.55 years. Group 2 represented a larger sample, including 87 females(41.6%) and 122 males(58.4%) with an average age of 55.82 years(Table 1). Both groups showed no statistical difference in age (P=0.910) or sex distribution (P=0.180). Comborbidities are listed in Table 2. Group 2 included a significantly higher number patients with liver disease(hepatitis, liver cirrhosis, and steatotic liver disease) (P=0.011), whereas other diseases showed no statistical significance.
2. Clinical Findings
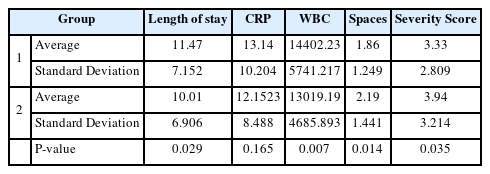
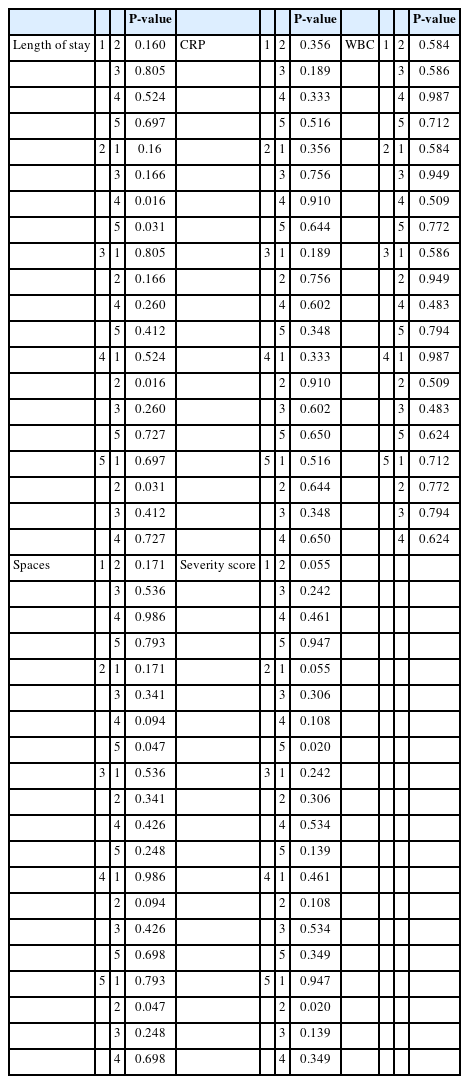
The clinical findings are summarized in Table 3, which shows the length of hospital stay, initial CRP levels, WBC counts, number of affected spaces, and severity scores. Length of hospital stay was longer in Group 1(11.47 ± 7.152) than that in Group 2 (10.60 ± 6.906) and showed statistical significance (P=0.029). The initial CRP levels were also higher in Group 1 than that in Group 2, but the difference was not statistically significant (P=0.165). The initial WBC counts were also significantly higher in Group 1 (P=0.007). Conversely, the number of affected spaces and severity scores were both higher in Group 2, showing a statistical difference in both categories (P=0.014 and P=0.035, respectively).
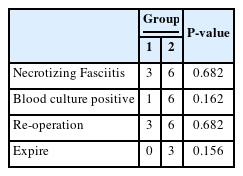
Other clinical signs and symptoms indicating the severity of the infections are listed in Table 4. Although Group 2 showed higher numbers in all categories of necrotizing fasciitis, blood culture positivity, reoperation, and expiration, none of them showed statistical significance.
3. Distribution of Affected Spaces
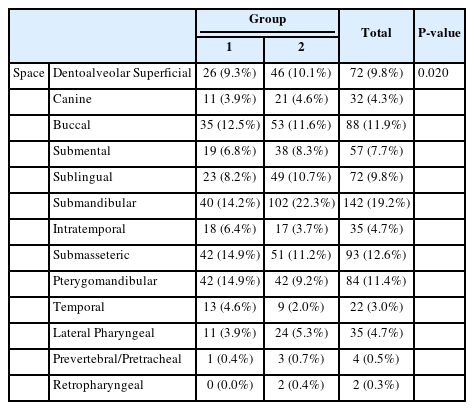
The distribution of affected spaces is shown in Table 5. Statistical significance was achieved in the submandibular, pterygomandibular, and temporal space categories. Group 1 had higher numbers in the pterygomandibular and temporal space categories, whereas Group 2 had higher numbers in the submandibular space category. As for deep neck space infections, represented by the lateral pharyngeal, retropharyngeal, prevertebral, and pretracheal spaces, Group 2 indicated ratio and number than that of Group 1; however, no statistical significance was achieved.
4. Treatment Modalities
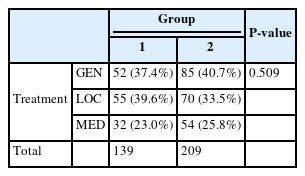
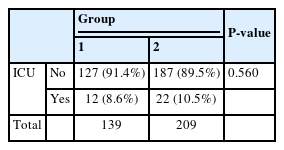
Each patient received surgical treatment or treatment with only medications. Among the patients who underwent surgical incision and drainage, surgery was performed either under general or local anesthesia based on the patient’s condition. The distribution of different treatment modalities is shown in Table 6-1. Both the ratio and number of patients who underwent surgery under general anesthesia were higher in Group 2(n = 85, 40.7%) than that in Group 1(n = 52, 37.4%); however, the difference was not statistically significant(P=0.509)
For severe cases, the patients underwent care in the ICU, and the number of patients admitted is depicted in Table 6-2. More patients were admitted to the ICU in Group 2(n = 22, 10.5%) than that in Group 1(n = 12, 8.6%); however, the difference was not statistically significant(P=0.560).
5. COVID-19 Negative and Positive Patients
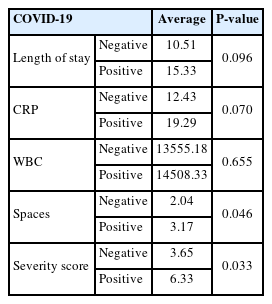
Six patients were tested positive for COVID-19. When comparing the parameters between COVID-19 positive and negative patients, COVID-19 patients showed higher values in every category; the specifics of which are presented in Table 7. Statistical significance was achieved for the number of affected spaces(P=0.046) and severity scores(P=0.033). Although the length of hospital stay and CRP levels were not statistically significant, P-values almost approximated to 0.05.
6. Different Waves of COVID-19
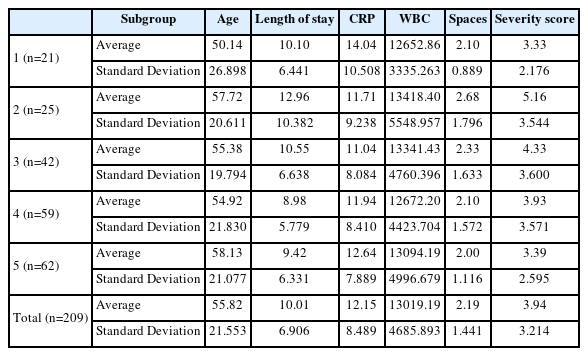
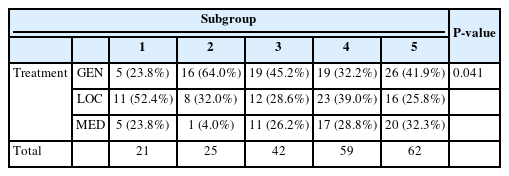
According to the KCDC, there were four different waves of the COVID-19 outbreak, and social distancing measures were completely resolved on April 19th, 2022. Hence, the five groups were subdivided within Group 2; the parameters for each subgroup are summarized in Table 8-1. The subgroup comprising the second wave of COVID-19 showed the highest values for every category, with CRP levels being an exception. P-values comparing each subgroup are presented in Table 8-3. Additionally, regarding the treatment modalities, which are presented in Table 8-2, Subgroup 2 showed the highest percentage of surgery under general anesthesia, which was statistically significant(P=0.041).
Ⅳ. Discussion
Odontogenic infections develop in the teeth or surrounding tissues, and when left untreated, they can lead to severe complications. The COVID-19 outbreak has become a global pandemic, resulting in more than 700million infections, 6million deaths worldwide as of 2023 according to the World Health Organization(WHO) [18,19]. Guidelines and policies have been implemented for the general public, including social distancing measures being one of them. Owing to a decline in foot traffic in public areas, private dental clinics suffered from decreased vistis [11,12]. This study was conducted under the premise that failure to address trivial dental problems on time will result in an increased number and severity of odontogenic infections in patients admitted to tertiary hospitals. Moreover, additional studies were performed to compare patients admitted during different COVID-19 waves and determine whether the actual COVID-19 infection complicated the odontogenic infection.
Group 1, which repented patients from the COVID-19 preceding years, comprised 139 patients, whereas Group 2 had 209 patients over the same time span of 3 years, showing a 50.4% increase. This result coincided with the studies conducted by Grill et al., Theim et al., and Samara et al., which implied that the emergency room(ER) burden of odontogenic infections increased because of the COVID-19 outbreak [13,14,20]. However there are studies with conflicting outcomes that reported a decrease in number of patients suffering from odontogenic infection [21,22]. Conversely, in Group 2, the length of hospital stay and initial CRP and WBC counts decreased compared with those in Group 1. However, this does not agree with the aforementioned studies. The difference in the length of hospital stay, although statistically significant, was only 0.87 days, and the patient’s clinical state on his or her discharge date was not always identical, implying that sometimes patients were discharged faster than others owing to factors such as being discharged against medical advice(AMA discharge) or stayed longer even if the acute phase of infection had subsided. Blood test values, particularly CRP levels and WBC counts, are routinely used to evaluate the severity of infection. These values are affected by several factors, including medication and the patients’ general health status. Medications that affect blood tests, include antibiotics and nonsteroidal anti-inflammatory drugs(NSAIDs), and medical conditions such as obesity, insomnia, depression, smoking, and diabetes, are also known to have an effect [23,24]. Although blood tests were performed when the patient presented to the ER before being administered intravenous or oral medication, it was difficult to evaluate the unaltered initial CRP levels and WBC counts, because, almost all patients were consuming some form of medication for pain inflicted by the infection, prior to visiting the ER.
Several conditions indicate the severity of infection. In this study, patients who showed signs of necrotizing fasciitis, positive blood culture results, need for reoperation, and death were recorded. Group 2 showed higher results in the four categories, but did not achieve any statistical significance. Several studies have reported similar results; however, none have showed statistical significance. This may be because of the fact that the four clinical features are quite rare, as the mortality rate of odontogenic infections had decreased drastically over the years [2,26].
The number of spaces affected was significantly higher in Group 2. In addition, when comparing the distribution of the affected spaces, an increase in the number of patients with the submandibular and deep neck spaces affected was observed in Group 2. Additionally, submandibular space was the most common space affected, yielding identical results with preceding studies [2,4,14]. The severity score was devised by Flynn et al., and the fascial spaces were rated using scores of 1, 2 or 3 according to their proximity to the airway and vital structures [4]. Group 2 showed significantly higher numbers of affected spaces and severity scores than Group 1. In a study by Fu et al. that determined the need for ICU admission for odontogenic infections, dysphagia, third molar infections, and CRP levels were the most relevant predictors [27]. One of the reasons why the severity score was devised was to evaluate the possible risk of odontogenic infections compromising the vital structures, including the airway. Group 2 had higher values in severity score, and ICU admissions corresponded to each other.
When dealing with patients with odontogenic infections, the surgeon can perform incision and drainage under general or local anesthesia, or treat conservatively using only antibiotics. Surgeries are normally performed under general anesthesia, or treat conservatively using only antibiotics. Surgeries are normally performed under general anesthesia in cases of severe infections [5]. Group 2 showed a slightly increased rate of surgeries under general anesthesia, which may imply that the severity in Group 2 was higher. Similar results have been obtained in previous studies, although statistical significance was not achieved in the current study [13,14].
Of the 209 patients admitted after the COVID-19 outbreak, only six tested positive for COVID-19. Theses six patients exhibited significantly higher values in every category than that exhibited by the remaining study participants. Studies have reported that the infection of COVID-19 might lead to opportunistic infections by other viruses, bacteria, and fungi, and vice versa. Additionally, some studies suggest that COVID-19 infection may lead to a change in the innate immune system, making it easier for other infections spread rapidly, and that infection with the virus itself can cause sepsis [16,26,28]. Even though the sample size was not large enough to validate this result, it is noteworthy that among the six patients, two developed sepsis, eventually expiring after being admitted to the ICU. Two other patients developed additional infections that formed distantly from the original location, and one of these became severe enough to require reoperation. Hence, further studies should include multiple centers to effectively evaluate the effect of getting infected to COVID-19 on complicated odontogenic infections.
Finally, to determine whether different social distancing measures had an effect on hospital admissions for odontogenic groups, Group 2 was subdivided into five subgroups. Among the different waves of the COVID-19 outbreak in Korea, the second wave had the highest fatality rate(1.88%). During this period, stricter infection control measures were implemented, such as the prohibition of using public spaces after 9 pm, non-face-to-face classes, and refraining from moving to other cities and provinces [10]. Accordingly, Subgroup 2, which represented patients admitted during the second wave, had the longest hospital stay, and highest values in WBC counts, number of affected spaces, severity score, and ratio for general anesthesia. This may imply that during the second wave of COVID-19, the severity of odontogenic infections was the highest.
This study has several limitations. First, the sample size was not large enough to produce significant results in several categories, particularly when comparing COVID-19 positive and negative patients, this problem is evident. Second, outpatients were not included in this study, which may have resulted in inaccurate outcomes when comparing parameters; however, this was intended so that only patients who were admitted for more than a day could be analyzed. Finally, when planning treatment for the patient, there was a difference in preference between different surgeons, indicating that even if different patients presented similar clinical features, there could be several ways to address the infection.
After the COVID-19 outbreak, the number of patients admitted to the Department of Oral and Maxillofacial Surgery at the Dankook University Hospital increased. Several parameters that represent the severity of the odontogenic infections also revealed an increase after the outbreak. Additionally, severity of the infection was the highest during the most critical wave of COVID-19. Finally, there was an increase in the severity of infection in COVID-19 positive patients.
To summarize, in the event of a worldwide pandemic, there could be alterations in the characteristics of patients with odontogenic infections, and healthcare professionals must be alert and prepared for such atypical circumstances.
Ⅴ. Conflicting Interests
The authors declare that they have no conflicting interests